Phthalimide is an important intermediate in organic synthesis, which can be used to produce phthalide, phthalonitrile, indigo, chlorhexidine and other fine chemicals. It is widely used in dye, pesticide, medicine, rubber and other industries. Il phthalimide is also an intermediate in the production of imidacloprid pesticide, which is an excellent variety for controlling pests in rice, cotton, vegetables and fruit trees because of its low toxicity and safe use.
The raw material phthalic anhydride is prepared by gas phase oxidation with o-xylene or naphthalene as raw material under the action of catalyst, so the whole reaction route is complex, polluting the environment and the overall yield is not high (taking the production of phthalimide in China as an example, the production of phthalic anhydride is mainly carried out by naphthalene oxidation method, with a molar yield of about 70%, and the yield of synthesizing imine from phthalic anhydride is 90%-95%, with a total molar yield of about 65%). We studied the catalytic reaction of o-xylene with ammonia and air to produce phthalimide in one step. The reaction is simple, the yield is high, and the method is environment-friendly.
The reaction equation is as follows:
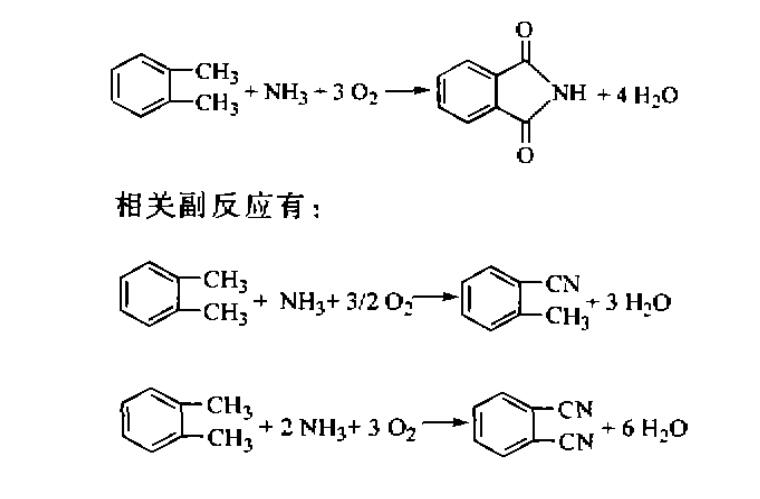
After tests, phthalimide manufacturers screened out N-86 catalyst with high efficiency, and found the appropriate process conditions. The product was obtained with high yield and very satisfactory purity.
(1) preparation of catalyst
The catalyst used for preparing phthalimides by ammoxidation of o-xylene is silica gel supported vanadium four-component metal oxide catalyst. The preparation method of the catalyst is impregnation method. Firstly, the oxide or salt of the required active component is added into distilled water according to a certain proportion, heated and dissolved to form a homogeneous solution, then poured into a metered silica gel, aged overnight, dried at 110℃, activated at 580℃ for 6 hours, naturally cooled, screened for later use, and recorded as N-86 catalyst;
(2) Synthesis of ortho-phthalimide
The reaction is carried out in a quartz tube fixed bed reactor with an inner diameter of 30ram: the reactor is filled with 15 n-No.86 catalysts, o-xylene (0.6 g, h) metered by micro pump is gasified, and then mixed with ammonia and air metered by rotameter, with ammonia flow rate of 0.61 l/h and air flow rate of 611/h; after preheating, the reaction mixture reacts through the catalyst bed.
The reaction temperature is maintained by heating from the south and controlled by a precise temperature controller, and the reaction temperature is 663±2K. The reacted product is condensed and deposited in a trap, and the tail gas is vented through a tail gas pipe: the trap is replaced every 8 hours, the product is washed out with water, dried and weighed, and 5.04 g of white needle crystals are obtained, with a raw material conversion rate of 98%, a molar yield of 75.7% and a selectivity of 77.2%; The purity of the product is 99.7% by gas chromatography, and the product contains 0.2% phthalonitrile, and the melting point is (234-235)℃.
II. Discussion
(1) Catalyst is the key to prepare phthalimide by ammoxidation. With different catalysts, different products I can be mainly produced. The N-86 catalyst studied by us has the characteristics of high efficiency and specificity for the formation of phthalimide, which not only has high product yield, but also has high purity. This study provides a good route and process for the synthesis of organic intermediate phthalimide. We intend to apply for patent for catalyst formula and process:
(2) Besides the catalyst, the reaction conditions have great influence on the reaction. Changing the reaction conditions can greatly affect the yield and purity of the product. Appropriate reaction temperature, higher air ratio and lower ammonia ratio are beneficial to the formation of phthalimide and can reduce the content of by-products phthalonitrile and o-toluonitrile.
(3) In view of the fact that o-xylene and ammonia as raw materials can produce phthalonitrile or phthalimide under different catalysts and appropriate adjustment of reaction conditions, in industrial production, it is considered to establish the same set of reaction equipment, arrange the production of different products and adjust the product structure according to market demand.