H NMR confirmed its structure, and measured its thermal stability, electrical conductivity and solubility in polyacrylonitrile. The experimental results show that most compounds have low melting points and are ionic liquids at room temperature. These compounds have good thermal stability and can be applied to the fields of electrodes and surfactants. And N- methyl -N- butyl morpholine hydrochloride has the dissolving ability to polyacrylonitrile.
Room temperature ionic liquids (RTILs) mainly refer to salts composed of organic cations and inorganic or organic anions which are liquid at room temperature or near room temperature, and have unique properties unmatched by many molecular solvents. Room temperature ionic liquid has low vapor pressure, wide range of liquid temperature and electrochemical window, high thermal stability and easy preparation, so its synthesis and application have become a research hotspot at home and abroad in recent years [1 ~ 4]. At present, most ionic liquids are composed of alkyl pyridine or dialkyl imidazole quaternary ammonium salt cation, chloroaluminate, fluoroborate, hexafluorophosphate and other anions.
However, due to the high cost of such ionic liquids, it is difficult to realize industrial production and application. Therefore, the design and synthesis of low-cost ionic liquids has become the focus of research. Recently, it has been reported that low-cost ionic liquids of morpholine quaternary ammonium salt cations are easy to prepare and have good electrochemical stability, and have been successfully used in electrochemical fields such as electrodes [5 ~ 7].
At present, the precursor morpholine quaternary ammonium salt synthesized for preparing morpholine ionic liquid is mainly methyl morpholine bromide salt. However, there are few studies on the physical and chemical properties of these new compounds. There is no report on the synthesis and properties of N-dialkyl morpholine hydrochloride ionic liquid. In addition, the study on the physical and chemical properties of new ionic liquid compounds can provide reference for the application of ionic liquids.
In order to further broaden the application of this kind of quaternary ammonium salt in ionic liquids, surfactants and electrodes, a series of new N- dialkyl morpholine hydrochlorides were synthesized from cheap N- methylmorpholine and N-ethylmorpholine, and their structures and physical properties were characterized by 1 HNMR, 13 C NMR, TG, DSC and elemental analysis, and their compatibility with conventional organic solvents was studied, and their solubility in polyacrylonitrile was tested. The experimental results show that these compounds have good thermal stability, and N- methyl -N- butyl morpholine hydrochloride can dissolve polyacrylonitrile well.
First, the experimental part
1, instruments and reagents
Automatic analyzer; Conductivity meter; Polarization microscope; Comprehensive thermal analyzer (STA 409 PC), DSC(Perkin Elmer DSC7), acetone, acetonitrile (analytically pure) and other solvents were dried by anhydrous calcium chloride for several days, and then added with metallic sodium to reflux and evaporate. Other reagents are analytically pure. 1。 2 synthesis
2. Compounds
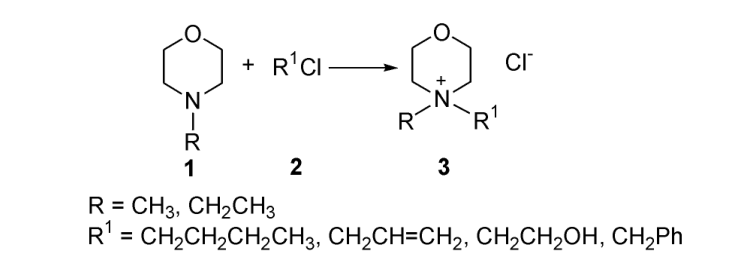
3a, 3b, 3e, 3g synthetic intermediate 1 (0. 1 mol) and intermediate 2 (0. 12 mol) into a 100mL three-necked flask, adding 20 mL acetonitrile as solvent, heating under magnetic stirring, controlling the reaction temperature, and evaporating the solvent with a rotary evaporator to obtain a crude product. The crude product was washed twice with ether, and recrystallized with acetone/ethanol mixed solvent to obtain the target compound. The physical and chemical data are shown in Table 1.
3. Synthesis of compounds 3c, 3d and 3f
Intermediate 1 (0. 1 mol) and intermediate 2 (0. 12 mol) into a 100mL three-necked flask, stirring at room temperature, filtering, washing the crude product twice with ether, and recrystallizing with acetone/ethanol mixed solvent to obtain the target compound, whose physical and chemical data are shown in Table 1.
4. Structural characterization of compounds 3A ~ 3G
3a: white solid, m. p。 90~91 ℃; 1 H NMR (300 MHz,CDCl 3 ) δ: 0。 96 (t, J=7。 2 Hz, 3H, CH 2 CH 3 ), 1。 41~1。 48(m, 2H, CH 2 ), 1。 69~1。 80 (m, 2H, CH 2 ), 3。 48 (s, 3H, CH 3 ),
3。 62~4。 12 (m, 10H); 13 C NMR (75 MHz, CDCl 3 ) δ: 13。 66(Bu-CH 3 ), 19。 64 (CH 2 ), 23。 69 (CH 2 ), 46。 99 (NCH 3 ), 59。 56(2C, NCH 2 ), 60。 75 (Bu-CH 2 N), 65。 09 (2C, OCH 2 )。
3b: white solid, m. p。 45。 3~46。 5 ℃; 1 H NMR (300MHz, D 2 O) δ: 3。 27 (s, 3H, CH 3 ), 3。 49~3。 53 (m, 2H, CH 2 ),3。 59~3。 65 (m, 4H, CH 2 NCH 2 ), 4。 02 (s, 6H, CH 2 OCH 2 ,CH 2 OH); 13 C NMR (75 MHz, DMSO) δ: 48。 17 (NCH3 ),54。 85 (2C, NCH 2 ), 60。 20 (CH 2 ), 60。 31 (CH 2 OH), 65。 09(2C, OCH 2 )。
3c: white solid, m. p。 101~102 ℃; 1 H NMR (300MHz, CDCl 3 ) δ: 3。 53 (s, 3H, CH 3 ), 3。 74 (s, 4H,CH 2 NCH 2 ), 4。 06 (s, 4H, CH 2 OCH 2 ), 4。 64 (d, J=6。 9 Hz,2H, CH 2 ), 5。 74~6。 12 (m, 3H, CH=CH 2 )。
3d: white solid, m. p。 151~152 ℃; 1 H NMR (300MHz, D 2 O) δ: 3。 08 (s, 3H, CH 3 ), 3。 34~3。 38 (m, 2H,CH 2 N), 3。 56~3。 59 (m, 2H, CH 2 N), 4。 02~4。 05 (m, 4H,CH 2 OCH 2 ), 4。 58 (s, 2H, CH 2 Ph), 7。 50~7。 56 (m, 5H, Ph); 13 C NMR (75 MHz, DMSO) δ: 45。 39 (NCH3 ), 58。 92 (2C,NCH 2 ), 60。 31 (2C, OCH 2 ), 67。 85 (CH 2 Ph), 127。 76[C(4)-Ph], 129。 34 [2C, C(3)-Ph], 130。 78 [2C, C(2)-Ph],
133。 71 [C(1)-Ph]。
3e: white solid, m. p。 53~54 ℃; 1 H NMR (300 MHz,DMSO) δ: 1。 23 (t, J=7。 06 Hz, 3H, CH 2 CH 3 ), 3。 39~3。 46(m, 4H, CH 2 NCH 2 ), 3。 48~3。 70 (m, 6H, CH 2 CH 3 , NCH 2 -CH 2 OH), 3。 92 (s, 4H, CH 2 OCH 2 ), 5。 74 (s, 1H, OH); 13 CNMR (DMSO, 75 MHz) δ: 7。 45 (CH 3 ), 46。 89 (CH 2 ), 55。 31(2C, NCH 2 ), 58。 23 (NCH 2 CH 2 OH), 60。 15 (CH 2 OH), 62。 03(2C, OCH 2 )。
3f: white solid, m. p。 97~99 ℃; 1 H NMR (300 MHz,D 2 O) δ: 1。 30 (t, J=6。 86 Hz, 3H, CH 2 CH 3 ), 3。 39~3。 51 (m,6H, CH 2 , CH 2 NCH 2 ), 4。 02~4。 04 (m, 6H, CH 2 OCH 2 ,NCH 2 CH=), 5。 65~5。 71 (m, 2H, =CH 2 ), 5。 87~5。 98 (m,1H, CH=);
13 C NMR (75 MHz, CDCl3 ) δ: 7。 60 (CH 3 ),54。 54 (CH 2 ), 56。 65 (2C, NCH 2 ), 60。 33 (2C, OCH 2 ), 60。 73(NCH 2 ), 123。 52 (CH), 129。 82 (=CH 2 )。
3g: white solid, m. p。 161~162 ℃,1 H NMR (300MHz, D 2 O) δ: 1。 43 (t, J=7。 13 Hz, 3H, CH 2 CH 3 ), 3。 39~
3。 51 (m, 6H, CH 2 , CH 2 NCH 2 ), 4。 03~4。 06 (m, 4H, CH 2 O-CH 2 ), 4。 59 (s, 2H, CH 2 -Ph), 7。 50~7。 55 (m, 5H, Ph); 13 CNMR (75 MHz, CDCl 3 ) δ: 8。 10 (CH 3 ), 52。 34 (CH 2 ), 55。 23(2C, NCH 2 ), 60。 35 (2C, OCH 2 ), 63。 89 (CH 2 Ph), 126。 58[C(4)-Ph], 129。 17 [2C, C(3)-Ph], 130。 65 [2C, C(2)-Ph],
133。 48 [C(1)-Ph]。
Second, the results and discussion
1. synthesis
In reference [8], the target compound 3c was synthesized by acetone and stirred at room temperature for 1 h, and the yield was 92%. The method has the characteristics of no organic solvent, short reaction time and high yield.
In addition, the reaction time has great influence on the reaction yield. Taking the synthesis of compound 3a as an example, the reaction time is 6 h, and the yield is very low, and almost no product can be obtained. When the reaction time is extended to 10 h, the yield is only about 20%, and when the reaction time is extended to 20 h, the yield is 42%. The reaction time was 30 h, and the yield increased little and hardly changed. Therefore, too long or too short reaction time is unfavorable to the reaction.
2. DSC analysis
By DSC test, the melting points of compounds 3a ~ 3g are 90 ~ 91℃ and 45℃, respectively. 3~46。 5 ℃, 101~102。 0 ℃, 151~152 ℃,53~54 ℃, 97~99 ℃, 161~162 ℃。 Except that the melting points of 3d and 3g are above 100℃, they are all ionic liquids, and the melting points are around 100℃ or below 100℃.
3. Influence of solvents on conductivity of ionic liquids
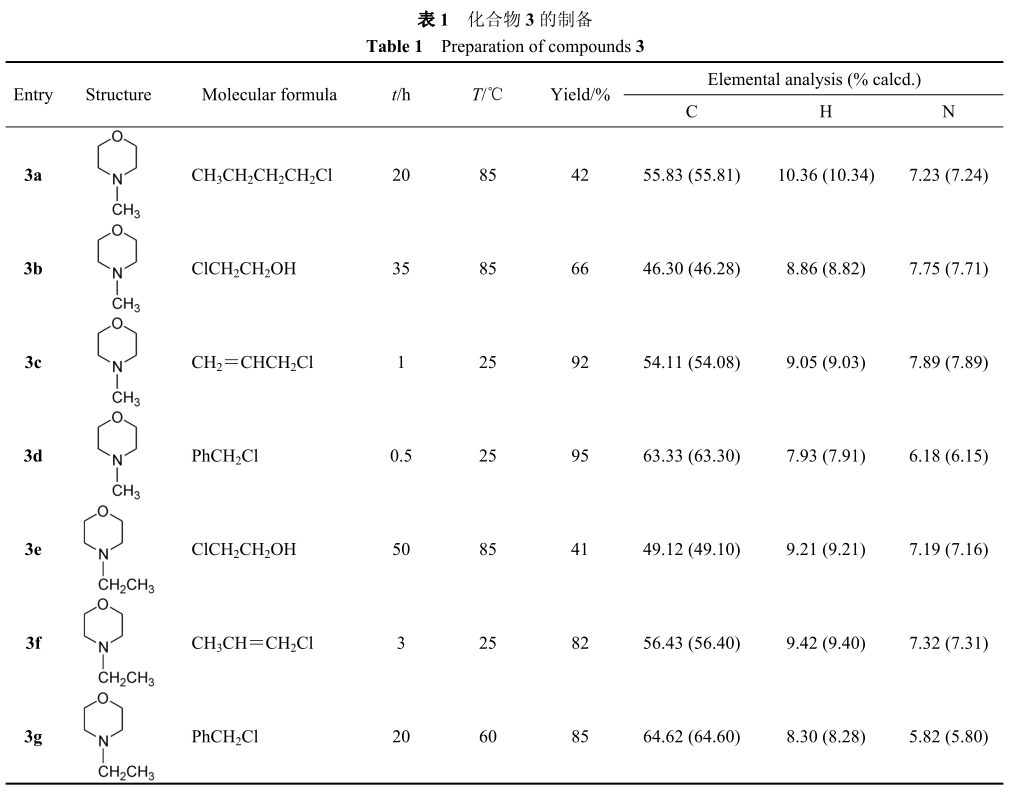
Fig. 1 shows the relationship between the concentration and conductivity of 3a-3g ionic liquids in different solvents such as water and absolute ethanol. It can be concluded from the figure that the conductivity of ionic liquids in different solvents is different, and there is a big difference. It can be seen from the figure that the conductivity of ionic liquid in water increases obviously with the increase of ionic liquid solution concentration; In absolute ethanol, the increasing trend of conductivity with increasing concentration is less than that with water as solvent. This is due to their different solvation in different solvents. The structure and polarity of solvents are different. Because of the different solvation effects of solvent molecules on anions and cations in different solvents, the solvation effects of solvents on ionic liquids are also quite different. The order of conductivity in different solvents is water > absolute ethanol.
4. Thermogravimetric analysis
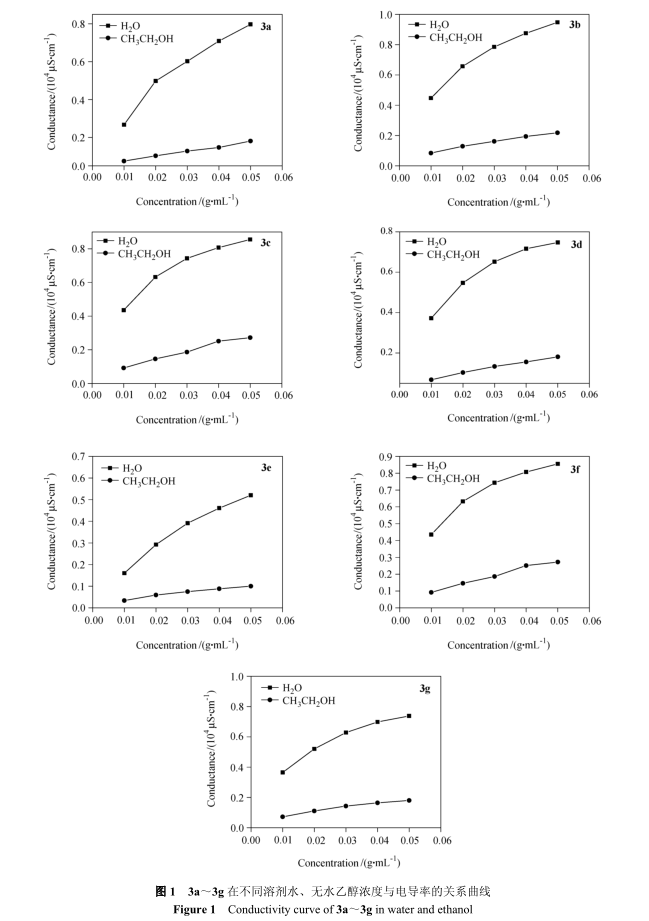
It can be seen from fig. 2 that the thermogravimetric temperatures of compounds 3a to 3g are 219. 3 ℃, 176。 9 ℃, 143。 1 ℃, 244。 7 ℃, 226。 4 ℃, 232。 6℃, 182。 7 ℃。 It shows that except 3b and 3c, the other six kinds of morpholine quaternary ammonium salt ionic liquids have high thermal stability.
5. Solubility analysis
The dissolution test results are shown in Table 2.
6. Observation on dissolution morphology of polyacrylonitrile in ionic liquid
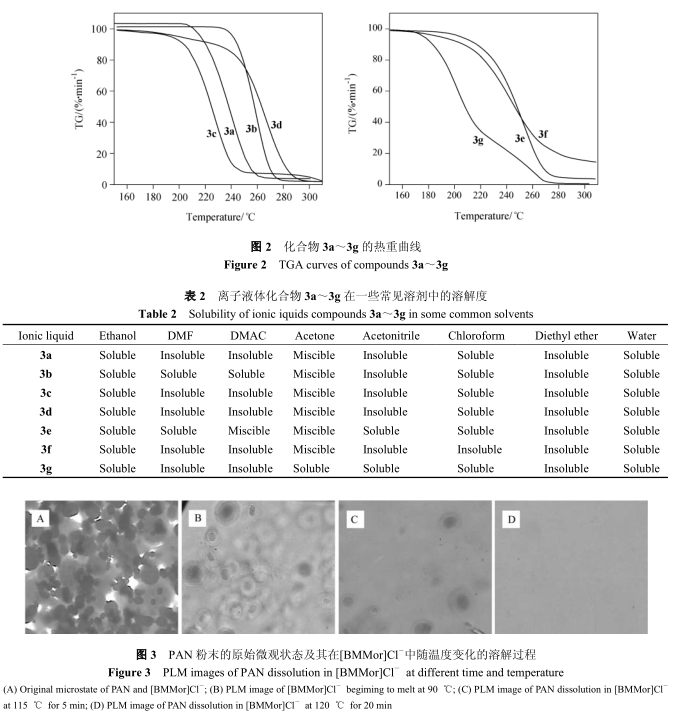
Ionic liquids are different from traditional solvents in that there are no molecules in their composition, but they are all ions. In ionic liquids, besides hydrogen bonds, dipole forces and Van der Waals forces existing in conventional solvents, there are also ionic bonds. Its special chemical structure will inevitably affect its interaction with solute polyacrylonitrile macromolecules, and even affect the morphological structure of polyacrylonitrile macromolecules and the interaction between polyacrylonitrile macromolecules. The following is the dissolution process of polyacrylonitrile in synthetic product 3a. Polyacrylonitrile is dissolved in ionic liquid to form a homogeneous and isotropic solution, while polyacrylonitrile which is not dissolved in ionic liquid will show a microscopic morphology with high brightness under orthogonal polarization.
Fig. 3 shows the original microscopic state of PAN powder and its swelling and dissolution process in ionic liquid [bmmor] cl-with temperature change.
Fig. 3A is the original microscopic state of ionic liquid and PAN powder under microscope, and fig. 3B is the initial microscopic state of PAN in [bmmor] cl-turbid solution when ionic liquid is completely melted at 90℃. Compared with fig. 3A, it can be seen in fig. 3B that the PAN powder particles are dispersed in [bmmor] cl-,which is in an irregular spherical shape. the darker part in the center of the particles is not wetted by ionic liquid [bmmor] cl-,on the contrary, the edge part has been wetted by ionic liquid. When the temperature rises to 115℃ and stays for 5 min, it can be seen that the dark part in the center of particles has been greatly reduced (see Figure 3C). When staying at 120℃ for 20 min, all particles disappeared and PAN powder was completely dissolved in ionic liquid [BMMOR] Cl-(see Figure 3D).