The dioxane manufacturer introduces the preparation process and properties of dioxane as a solvent in the highly dispersed copper/magnesium-aluminum composite oxide catalyst!
On the basis of preparing MgO-Al 2 O 3 composite oxides with similar specific surface area and adjustable alkalinity, we need to realize the uniform dispersion of copper on these carriers. Aqueous solution impregnation is one of the most commonly used preparation methods of supported catalysts. However, when the alkaline oxide is used as the carrier, such as MgO and MgO-Al 2 O 3 composite oxide, the aqueous solution is alkaline after contacting with the oxide, which makes the metal cations in the aqueous solution quickly converted into hydroxide precipitates and can not be uniformly adsorbed on the carrier surface, resulting in serious aggregation of the finally obtained metal particles.
This situation is very obvious when Cu 2++is used as metal source [12]. Here, Cu/MgO-Al _ 2 O _ 3 catalyst is prepared by ethanol solution of copper acetate instead of common aqueous solution, so as to realize the high dispersion of metal Cu on the surface of alkaline carrier. As shown in fig. 1, the x-ray diffraction pattern of Cu/MgO-Al 2 O 3 (3/1) catalyst only shows the characteristic diffraction peak of cubic MgO (2θ = 36) in the range of 2 ~ 6 wt% Cu loading. 9°, 42。 9°,62。 3) without the diffraction peak corresponding to Cu. This implies that Cu is highly dispersed on MgO-Al 2 O 3 (3/1) carrier. On the contrary, the characteristic diffraction peak of Cu(200) crystal plane (2θ = 50) can be clearly observed on 4 wt%Cu/MgO-Al 2 O 3 (3/1) catalyst when leaching with water solution of copper acetate. 4°)。 According to the results of N 2 O chemisorption experiment, the Cu dispersion of Cu/MgO-Al 2 O 3 (3/1) catalyst is about 0 when the Cu loading is 2 ~ 6 wt%. 83 (Table 2), that is, when Cu is highly dispersed, the dispersion degree of Cu particles on the catalyst surface is basically not affected by Cu loading. However, for the 4 wt% Cu/MgO-Al _ 2 O _ 3 (3/1) catalyst obtained by aqueous solution impregnation method, the dispersion degree of Cu is only 0. 14。
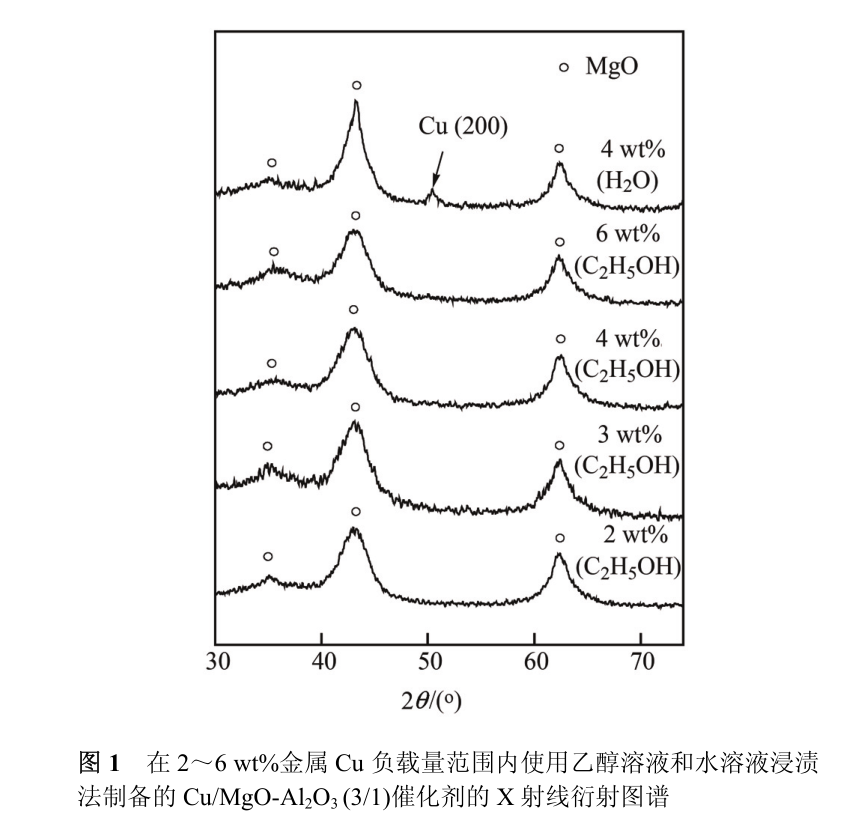
The above experimental results show that the high dispersion of Cu on MgO-Al _ 2 O _ 3 carrier can be effectively realized by ethanol solution impregnation method. In addition, it is also noted that the interaction between water and MgO-Al 2 O 3 composite oxide will lead to the reconstruction of MgO-Al 2 O 3 composite oxide, which makes the surface area of the catalyst significantly decreased compared with that of the fresh carrier after immersion in aqueous solution, while the interaction between ethanol and MgO-Al 2 O 3 composite oxide is much smaller, which makes the carrier keep the original composite oxide structure better by ethanol solution immersion method.
In addition to the dispersion degree of Cu, the dehydrogenation ability of Cu particle surface is another major factor affecting the hydrogenolysis activity of glycerol. The latter depends not only on the structure of Cu particles, but also on the interaction between Cu and the carrier. N _ 2 O chemisorption-H _ 2 temperature programmed reduction is an effective method to characterize the dehydrogenation ability of Cu particles. In a suitable temperature range, N 2 O will dissociate and adsorb on Cu surface to form stoichiometric adsorbed oxygen atoms [22]. Furthermore, when the adsorbed oxygen atoms are titrated by H 2 temperature-programmed reduction process, the reduction temperature of oxygen atoms reflects the dissociation and activation ability of Cu particle surface to H 2.
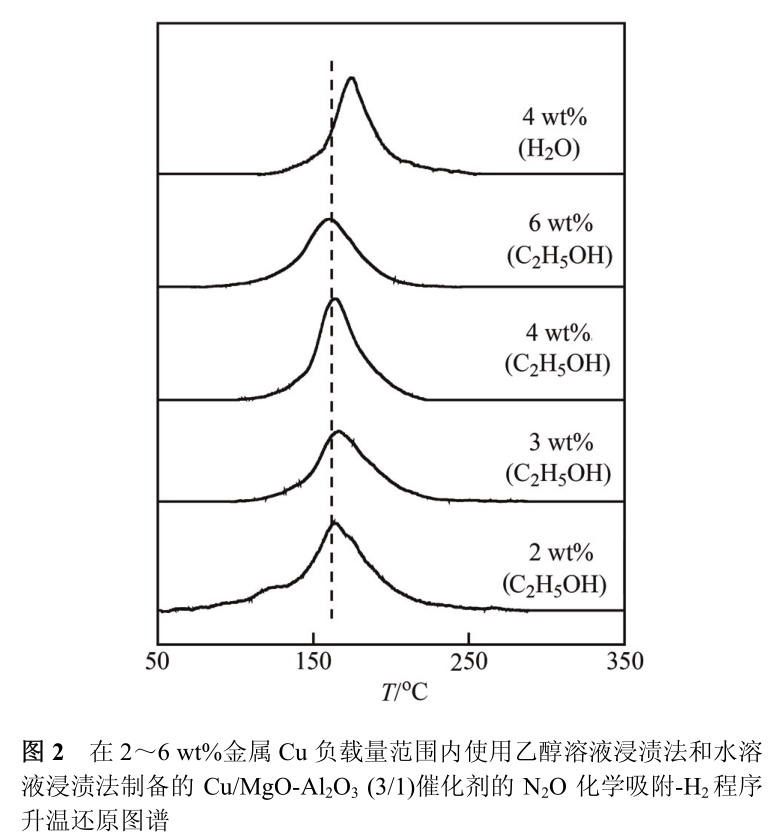
According to the research results of specialized chemical plants, the dissociation and activation ability of Cu particles to H 2 is related to its dehydrogenation ability [8]. The lower the reduction temperature of adsorbed oxygen atoms, the stronger the H 2 dissociation ability and the corresponding dehydrogenation ability on the surface of Cu particles. Fig. 2 shows the H 2 temperature-programmed reduction graph of oxygen atoms adsorbed on the surface of Cu/MgO-Al 2 O 3 (3/1) catalyst with Cu loading of 2, 3, 4 and 6 wt% respectively. Oxygen atoms adsorbed on the surface of the above four samples all show a single reduction peak near 164℃.
This suggests that Cu particles are uniformly dispersed on the surface of MgO-Al _ 2 O _ 3 composite oxide, and have similar H _ 2 dissociation ability and dehydrogenation ability. In contrast, the 4 wt% Cu/MgO-Al _ 2 O _ 3 (3/1) samples obtained by immersion in aqueous solution have lower dispersion of Cu, and the corresponding reduction peak of adsorbed oxygen atoms on the surface also appears at a higher temperature of 175℃. The above results are consistent with the conclusion that Cu-ZnO binary catalyst has higher hydrogen dissociation ability and dehydrogenation ability.
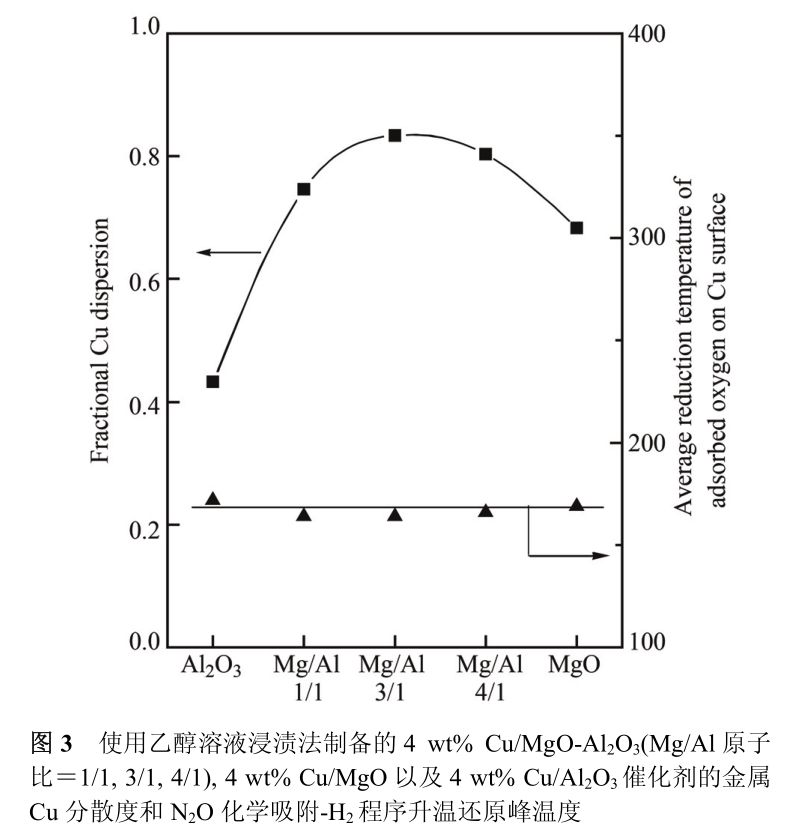
The ethanol solution impregnation method can not only achieve high dispersion of Cu on MgO-Al 2 O 3 (3/1) composite oxide, but also extend to other carriers such as Al 2 O 3, MgO-Al 2 O 3 (1/1), MgO-Al 2 O 3 (4/1) and MgO. As shown in fig. 3, on the premise of keeping Cu loading at 4 wt%, Cu on MgO-Al 2 O 3 (1/1) and MgO-Al 2 O 3 (4/1) composite oxides has similar dispersivity to MgO-Al 2 O 3 (3/1), which is 0 respectively. 75 and 0. 80, that is, the ratio of Mg/Al in MgO-Al _ 2 O _ 3 composite oxide does not significantly affect the highly dispersed state of Cu. On the pure MgO carrier, the dispersion degree of Cu particles decreased slightly to 0. 68, which may be attributed to the relatively low specific surface area of MgO (163 m 2 /g). For pure Al 2 O 3 carrier, although Al 2 O 3 and MgO-Al 2 O 3 composite oxide have similar surface areas (309 ~ 384 m 2/g), the dispersion degree of Cu particles on Al 2 O 3 carrier is only 0. 43, even lower than the dispersion result of Cu particles on MgO carrier.
This phenomenon suggests that the strong adsorption of Cu 2++on Lewis basic sites on MgO surface may play a key role in the dispersion degree and stability of the finally formed Cu particles. On the other hand, the results of N 2 O chemisorption-H _ 2 temperature-programmed reduction experiments show that the adsorbed oxygen atoms on the above five copper-based catalysts have similar H _ 2 reduction temperatures (164 ~ 172℃, Figure 3), so the intrinsic dehydrogenation ability of Cu particles in the above catalysts is similar, that is, there is no strong interaction between the carrier and Cu, and the change of carrier composition will not significantly affect the D electronic state of Cu particles.
Based on the above results, highly dispersed Cu/MgO-Al _ 2 O _ 3 composite oxide catalyst was synthesized by ethanol solution impregnation method, and the intrinsic dehydrogenation ability of Cu particles in the catalyst was not affected by Cu loading and carrier composition. Further investigation on the hydrogenolysis performance of glycerol over the above catalysts will help us to know more accurately the synergistic effect between basic center and metal center in hydrogenolysis of glycerol.