Ensuring the stability of the catalyst structure in the reaction process is the basic premise to measure the catalyst activity. Glycerol hydrogenolysis reaction is usually carried out at 160 ~ 220℃ and certain H 2 pressure. When water is chosen as the solvent for hydrogenolysis of glycerol, the hydrothermal environment formed under the reaction conditions requires strict stability of the catalyst. For example, Cu particles are easy to aggregate during the reaction [23]. Bienholz et al. [24] recently found that the use of non-aqueous solvents can effectively avoid the above problems.
Actually, the hydrogenolysis reaction of glycerol can also be effectively carried out in polar organic solvents such as dioxane [6], sulfolane [6] and alcohols [24]. Here, dioxane is selected as the solvent for hydrogenolysis of glycerol. On the one hand, it is used to keep the catalyst structure stable in the reaction process, and on the other hand, it can minimize the influence of MgO-Al 2 O 3 composite oxide on the hydrogenolysis activity of glycerol by changing the pH value of the solution. Firstly, we investigated the influence of carrier composition on the hydrogenolysis activity of glycerol over Cu/MgO-Al 2 O 3 catalyst (Table 3).
At about 25% glycerol conversion, 4 wt% Cu/Al _ 2 O _ 3 catalyst has very low glycerol hydrogenolysis activity, and its corresponding glycerol conversion rate is only 0. 1 mol/mol surface Cu-s。 After MgO is introduced into the carrier, the activity of the catalyst is obviously improved. The glycerol conversion rate of 4 wt%Cu/MgO-Al 2 O 3 (1/1) catalyst is 1. The activity of 6mol/mol surface Cu-s is 16 times that of 4 wt% Cu/Al 2 O 3 catalyst. With the increase of MgO content in the support, the hydrogenolysis activity of glycerol of Cu/MgO-Al _ 2 O _ 3 catalyst also increased. Among the five catalysts studied, 4 wt% Cu/MgO showed glycerol conversion rate (2. 4 mol/mol surface Cu-s)。 Although the hydrogenolysis activity of the above catalysts varies significantly with the composition of the support, their selectivity for hydrogenolysis of glycerol is basically the same. 1,2-propanediol is the main product of the reaction, with selectivity ranging from 90% to 94%, and its corresponding main by-product is ethylene glycol, with selectivity kept at 5% to 8%. The results are similar to the selectivity of copper-based catalyst for hydrogenolysis of glycerol in aqueous solution. This suggests that using dioxane instead of water as solvent does not change the path of hydrogenolysis reaction of glycerol, and dehydrogenation of glycerol to glyceraldehyde on the surface of metal Cu catalyst is still the decisive step of the reaction.
Considering that the intrinsic dehydrogenation activities of Cu particles in the above catalysts are very close, and the basic site density on the surface of the support increases with the increase of Mg/Al ratio in MgO-Al 2 O 3 composite oxide (Table 1), the change of hydrogenolysis activity of the catalyst with MgO content in the support should be due to the cocatalysis of the basic center on MgO on the dehydrogenation process of glycerol.
In addition, when 4 wt%Cu/Al 2 O 3 catalyst was used, mechanical mixing of a certain amount of MgO-Al 2 O 3(3/1) composite oxide did not change the activity and selectivity of the original catalyst, which indicated that the basic center in Cu/MgO-Al 2 O 3 catalyst directly promoted the hydrogenolysis activity of Cu, but not indirectly affected the conversion rate of glycerol by changing the OH-concentration of the reaction system.
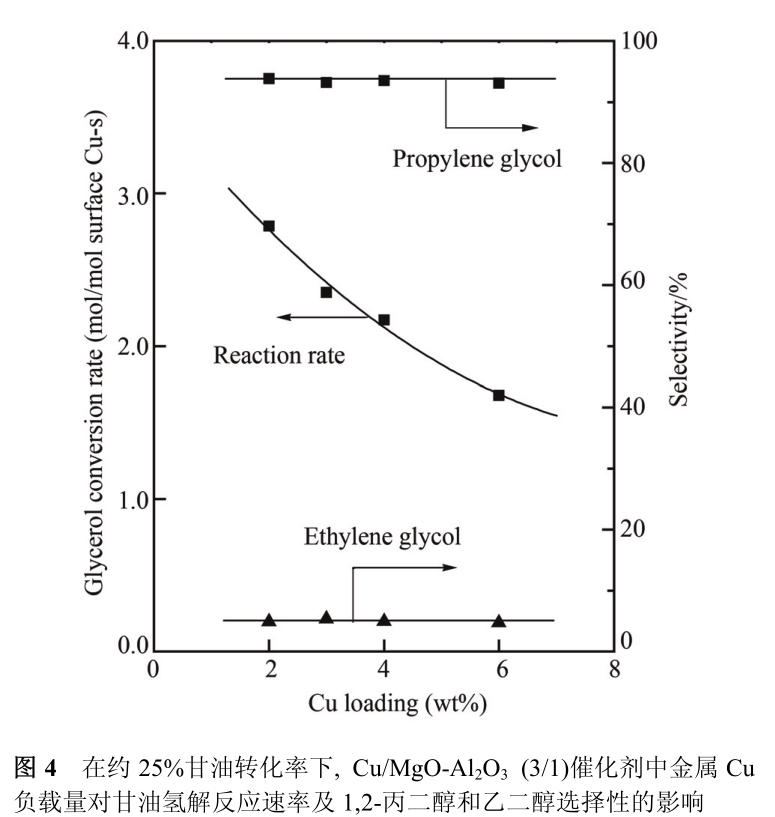
In order to further confirm the synergistic effect between basic center and metal center in Cu/MgO-Al _ 2 O _ 3 catalyst, we investigated the effect of Cu loading on glycerol hydrogenolysis activity of Cu/MgO-Al _ 2 O _ 3 (3/1) catalyst. As shown in fig. 4, with the increase of Cu loading in Cu/MgO-Al 2 O 3(3/1) catalyst from 2 wt% to 6 wt%, the conversion rate of glycerol is from 2. The 8 mol/mol surface Cu-s decreased to 1. 7 mol/molsurface Cu-s, but the selectivity of 1,2-propanediol and ethylene glycol has no obvious change. Since the intrinsic dehydrogenation activity of Cu particles in the catalyst does not change with the loading of Cu, the decrease of Cu atom activity per unit surface with increasing Cu loading can be attributed to the decrease of the number of accessible basic sites of Cu atoms on the catalyst surface.
Therefore, the two groups of experiments of adjusting the density of basic centers and the density of metal centers in Cu/MgO-Al _ 2 O _ 3 catalyst respectively confirmed that the basic centers and metal centers on Cu/MgO-Al _ 2 O _ 3 catalyst cooperated to complete the dehydrogenation of glycerol to glyceraldehyde in the hydrogenolysis reaction of glycerol. The synergistic effect between basic sites and metal sites can be quantitatively described by the ratio of basic sites to Cu atoms on the surface of Cu/MgO-Al _ 2 O _ 3 catalyst.
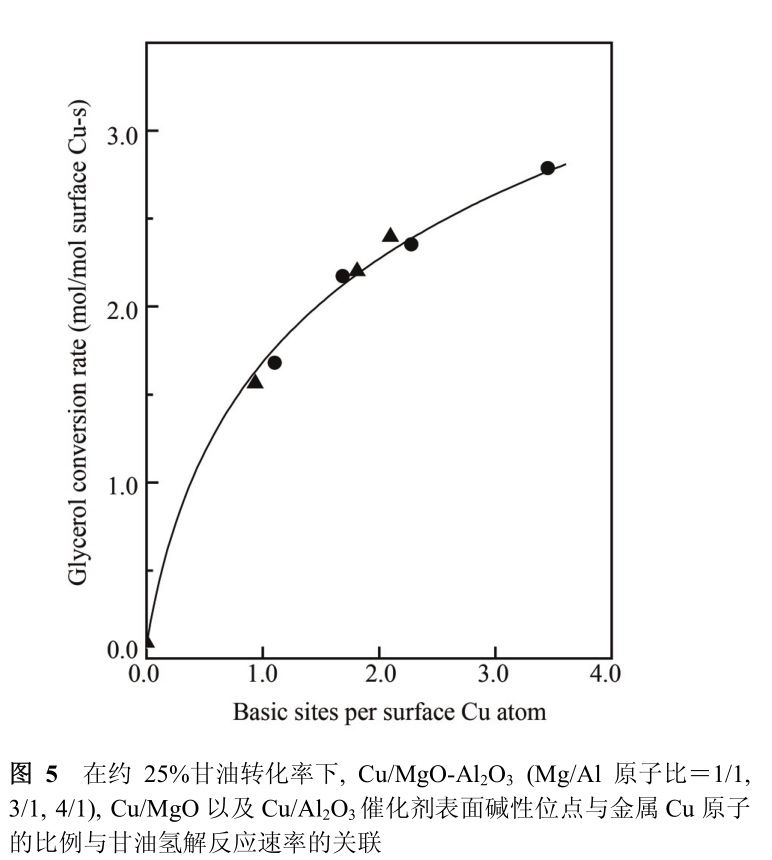
Based on the above experimental results of two groups of Huai 'an Chemical Factory in Figure 5, it can be seen that the hydrogenolysis activity of glycerol of Cu/MgO-Al 2 O 3 catalyst increases with the increase of the ratio of basic sites on the catalyst surface to Cu atoms, and there is a good correlation between them. It is worth noting that the glycerol hydrogenolysis activity of Cu/MgO-Al _ 2 O _ 3 catalyst slows down with the increase of the ratio of basic sites to Cu atoms on the catalyst surface.
This suggests that when there are sufficient accessible basic sites around Cu particles on the catalyst surface, the hydrogenolysis activity of glycerol of the catalyst mainly depends on the dehydrogenation ability of Cu particles themselves. In fig. 5, the glycerol hydrogenolysis activity of Cu/MgO-al _ 2 o _ 3 catalyst has a good correlation with the ratio of basic sites on the catalyst surface to metal Cu atoms, suggesting that the surfaces of Cu/MgO-al _ 2 o _ 3 catalysts with different Mg/Al ratios have the same basic sites, which are derived from o _ 2-ions bonded with mg ~ (2+). Considering that a small amount of water left in the reaction system and water generated in the hydrogenolysis of glycerol will hydroxylate the surface of MgO-Al 2 O 3 carrier [25, 26].
Therefore, with the hydrogenolysis of glycerol, the basic sites on the surface of Cu/MgO-Al _ 2 O _ 3 catalyst will change from O _ 2-ion to hydroxyl (B-base OH-).
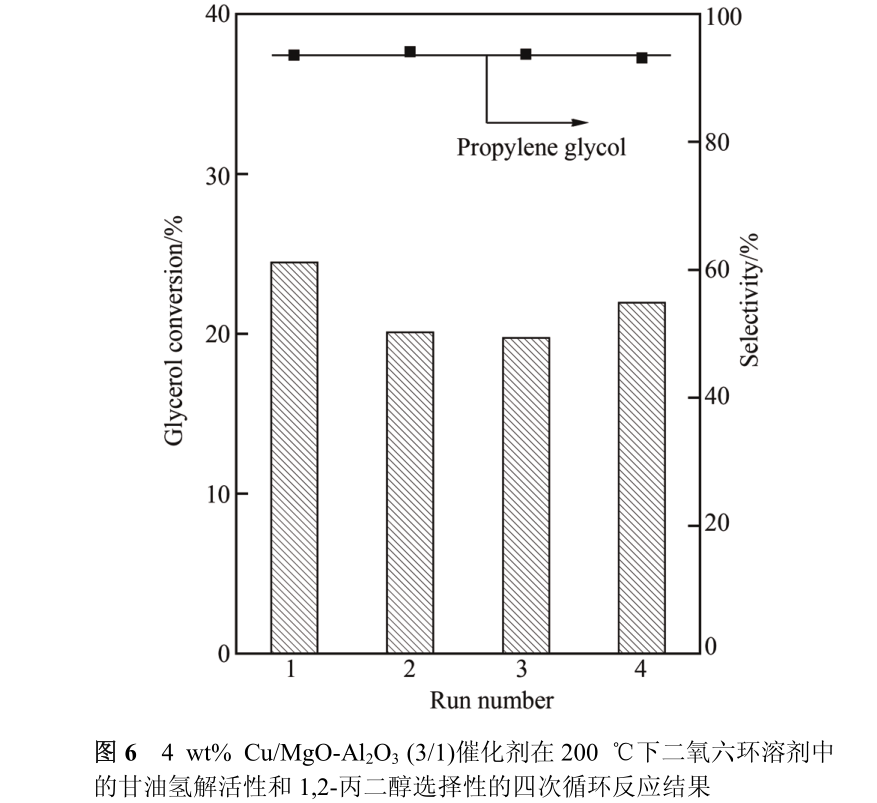
The stability of 4 wt% Cu/MgO-Al _ 2 O _ 3 (3/1) catalyst showed that after four reaction cycles, the activity and selectivity of the catalyst for hydrogenolysis of glycerol did not change significantly (Figure 6). We speculate that the hydroxylation of the catalyst surface occurred at the initial stage of the reaction, so the hydroxyl group bonded with Mg ~ (2+) on the catalyst surface is the basic center actually participating in the hydrogenolysis of glycerol.
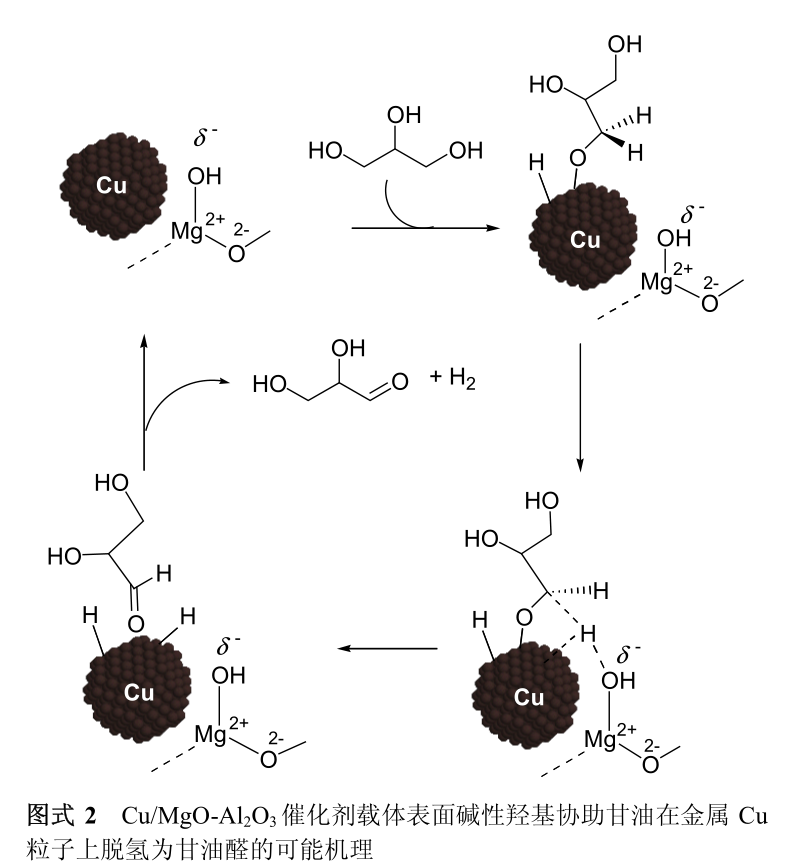
The possible mechanism that the basic hydroxyl groups on the surface of Cu/MgO-Al _ 2 O _ 3 catalyst assist Cu particles to complete dehydrogenation of glycerol to glyceraldehyde is shown in Figure 2: First, glycerol molecules are dissociated and adsorbed on the surface of Cu as alcoholoxy groups and hydrogen atoms. The alcoholoxy group and the basic hydroxyl group at the interface between Cu and the carrier have induced polarization, which weakens the C-H bond at α position of the alcoholoxy group. The weakened C-H bond at α-position of alcoholoxy was broken on the surface of Cu, and finally glyceraldehyde and a molecule of H 2 were produced.