3-(2- nitrophenoxy) -1- propylamine was synthesized by reacting 2- nitrophenol sodium with N-(3- bromopropyl) phthalimide in anhydrous DMF with KI as catalyst to synthesize 2-[3-(2- nitrophenoxy)-propyl] dihydroisoindole -1,3- dione (4a), and then
Since N-(3- bromopropyl) phthalimide and phthalimide in the product can be hydrolyzed under alkaline conditions, the reaction cannot be carried out in water, and anhydrous solvent should be used. The method has high operational requirements and expensive reagents. We prepared 3- aryloxy -1- bromopropane from substituted 2- nitrophenol and 1,3- dibromopropane with NaOH as base, and then synthesized 3-aryloxy -1- propylamine through Gabriel reaction, and the reaction conditions were studied. The reaction of substituted 2- nitrophenol with 1,3-dibromopropane can be carried out under conventional heating and microwave radiation heating [13].
The results showed that the reaction was completed in 5 hours under conventional heating and only 10 minutes under microwave radiation heating, which obviously accelerated the reaction speed and improved the yield. According to the literature, when synthesized by conventional heating method, the reaction solution is two-phase. The reaction is accelerated by vigorous stirring and PTC addition, but the hydrolysis of 1,3- dibromopropane and 3-aryloxy -1- bromopropane is also accelerated, which affects the reaction yield. It was found that the substituted 2- nitrophenol sodium was prepared in a small amount of water, and then 1,3-dibromopropane and tetrabutylammonium bromide were added. After ultrasonic dispersion, the mixture was placed in a microwave reactor with a power of 130W and irradiation for 10min, and the reaction was finished with high yield.
The reaction speed is slow when the power is 65W, and polymer is formed when the power is 300W. Because of the small amount of water, the reaction is basically carried out without solvent, which can not only speed up the reaction, but also reduce the hydrolysis of raw materials and products and increase the yield. 1,3-dibromopropane has two substitution reaction points, which can form disubstituted products with substituted sodium nitrophenolate. Therefore, the amount of 1,3-dibromopropane should be increased during the reaction to reduce the formation of disubstituted products. When the molar ratio of substituted nitrophenol to 1,3-dibromopropane is 1∶2, only a small amount of disubstituted products are formed during conventional heating and microwave heating reaction.

In the synthesis of compounds 5a ~ 5f, the white flocculent precipitate produced by hydrazinolysis is insoluble in chloroform. After filtering, evaporating ethanol under reduced pressure, adding chloroform to dissolve, filtering, and washing with water, 3- aryloxy -1- propylamine can be obtained. When the hydrochloride is formed, add a proper amount of concentrated hydrochloric acid into chloroform solution, shake well, evaporate chloroform under reduced pressure, and add acetone to disperse the residue to obtain the corresponding hydrochloride.
Compounds 4a ~ 4f failed to obtain molecular ion peaks when HRMS(EI) was determined, and all compounds 4a ~ 4f gave fragment ion peaks of m/z180 and 160, which was consistent with the structure of compounds which were easy to crack at β position of nitrogen atom and α position of aryl ether oxygen atom in phthalimide fragment, and formed fragment ion peaks containing phthalimide fragment. The results of 4a ~ 4f elemental analysis are completely consistent with the structure of the compound. The structures of all compounds were confirmed by FTIR, 1HNMR, 13CNMR, HRMS or elemental analysis.
Discussion on biological activity and influencing factors of target compounds
In order to determine the inhibitory activity of the target compound in vitro, it was determined by ELISA that different concentrations of the target compound and biotinylatedTat-AcK50 histidine combined competitively with GST-fusionBRD immobilized in a 96-well plate. The measurement results are shown in Table 1. The results of in vitro inhibitory activity test showed that compounds 5a-5f could inhibit the activity of HIV-1AcTat at higher concentrations. However, compared with N1-(2- nitroaryl)-1,3-propanediamine hydrochloride, its inhibitory activity in vitro decreased significantly.
On the basis of a detailed analysis of the 3D structure of the complex of N1-(4- methyl -2- nitrophenyl)-1,3-propanediamine hydrochloride (1b) and PCAFBRD, it is considered that the inhibitory activity of this compound mainly comes from hydrogen bonding between 2- nitro group and phenolic hydroxyl groups of Y802 and Y809 (D1 = 2. 85Å,d2=3。 09), and the salt bridge formed by the ammonium salt ion at the end of 1,3-propanediamine branch chain and the carboxyl ion of E750 (d = 2. 92) (fig. 2A). The 2-nitro group is located at the cross position with benzene ring, which is beneficial to form hydrogen bonds with phenolic hydroxyl groups of protein amino acid residues Y802 and Y809 in target sites.
In order to analyze the reasons for the decline of biological activity of compounds 5a ~ 5f, we calculated the dominant conformations of compounds 1b and 5b by software. The results showed that the ammonium salt ions at the end of 1,3-propanediamine branched chain formed hydrogen bonds with the 2-nitro group. But hydrogen bonding in compound 5b (d = 1. 88) (fig. 2B) is stronger than compound 1b (d = 2. 25) (fig. 2C).
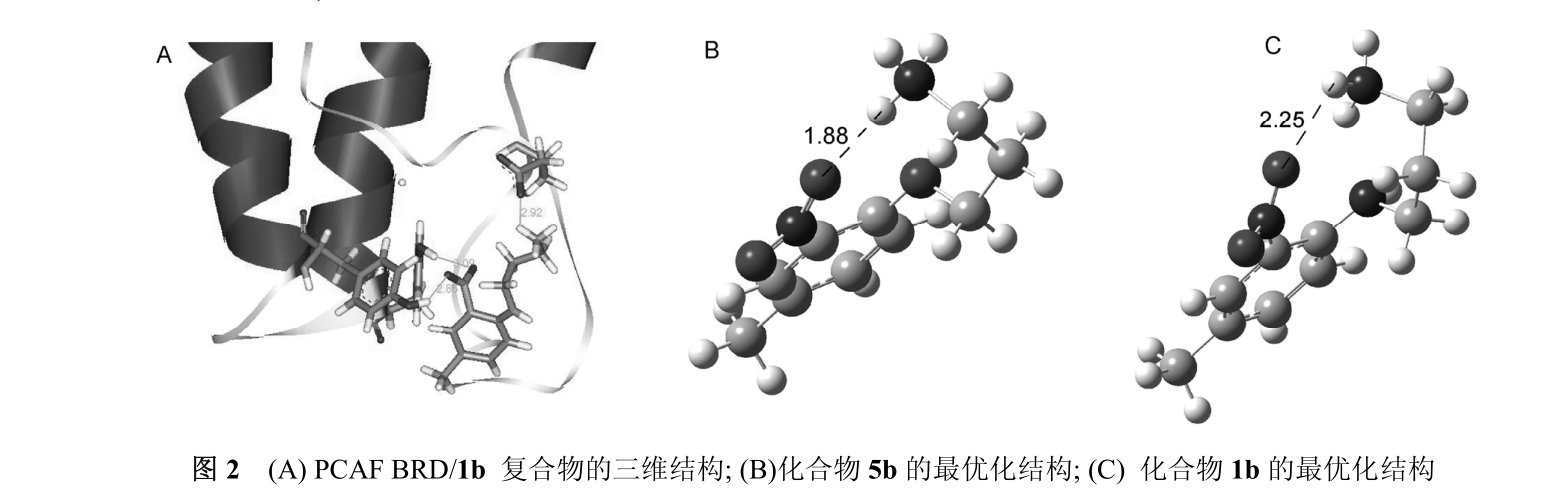
When the compound molecules enter the protein target and interact with the amino acid residues in the protein, the hydrogen bond formed by the ammonium salt ions at the end of 1,3-propanediamine branches in 1b and 5b and the 2-nitro group should be destroyed first. Due to the strong hydrogen bond in compound 5b, it is difficult to break it, which affects the interaction between the compound and the amino acid residues in the target protein.
At the same time, the hydrogen bonding also affects the spatial orientation of the 2-nitro group, which makes it almost coplanar with the benzene ring, and it is difficult to form hydrogen bonding with the phenolic hydroxyl groups of the protein amino acid residues Y802 and Y809 in the target at the same time. These factors may be the main reasons for the decrease of the activity of 3- aryloxy -1- propylamine hydrochloride.
Studies have shown that N1-(2- nitroaryl) -1,3- propanediamine hydrochloride has high structural specificity as an inhibitor of HIV-1Tat/PCAFBRD, and its activity decreases when it is changed into 3-aryloxy -1- propanamine hydrochloride. The research has certain guiding significance for further optimization design of HIV-1Tat/PCAFBRD inhibitors.