Cancer is one of the most serious social health problems in the world. Synthetic death is a very good cancer treatment strategy. In 2014, FDA approved Olapaib for the treatment of patients with advanced ovarian cancer who had received more than two previous treatments and carried germline BRCA mutation. OLAP AIB is a cancer therapy targeting Poly ADP-ribose Polymerase, and it is also an anticancer drug successfully approved for clinical use by using the concept of Synthetic Lethality.
The main biological function of PARP-1 is to participate in the damage repair process of single-strand DNA breaks,SSBs), and the main biological function of BRCA is to participate in the damage repair process of double-strandDNA breaks (DSBs). When both repair processes are inhibited, the DNA repair process will be blocked, thus accumulating more broken DNA and eventually causing cell death.
Phthalimide derivatives usually exhibit various biological activities, so structural modification of phthalimide molecules has been widely studied in recent years. Derivatives substituted on imide nitrogen are condensation molecules of phthalic anhydride and primary amines, which can be used as angiogenesis inhibitors and immunomodulators.
PARP-1 inhibitors are usually carbamylated benzoheterocyclic derivatives, and benzamide is an essential active structure for inhibiting this enzyme. NMS-P118 is an effective inhibitor of PARP-1 with oral activity and high selectivity. It has good drug absorption and pharmacokinetic characteristics, and its selectivity for PARP-1 is 150 times higher than that for PARP-2. Therefore, we designed a series of compounds with N- substituted phthalimide -4- formamide as the mother core (Chart 1) based on PARP-1 inhibitor NMS-P118.
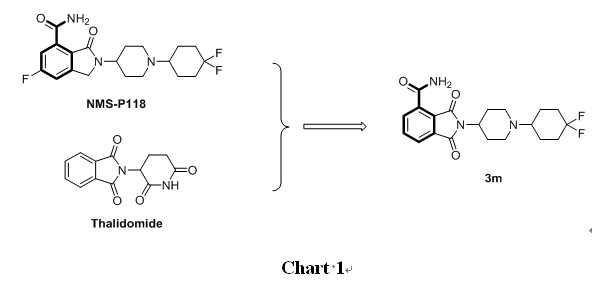
Brca1/2 mutant cancer cell lines are very sensitive to PARP-1 inhibitors, because inhibiting PARP-1 at the same time when Brca1/2 is deficient will cause synthetic death and promote cancer cell death. Therefore, Brca2 mutant pancreatic cancer cell line Capan-1 and Brca1/2 non-mutant breast cancer cell line MDA-MB-231 were used to test the activity of the compounds.
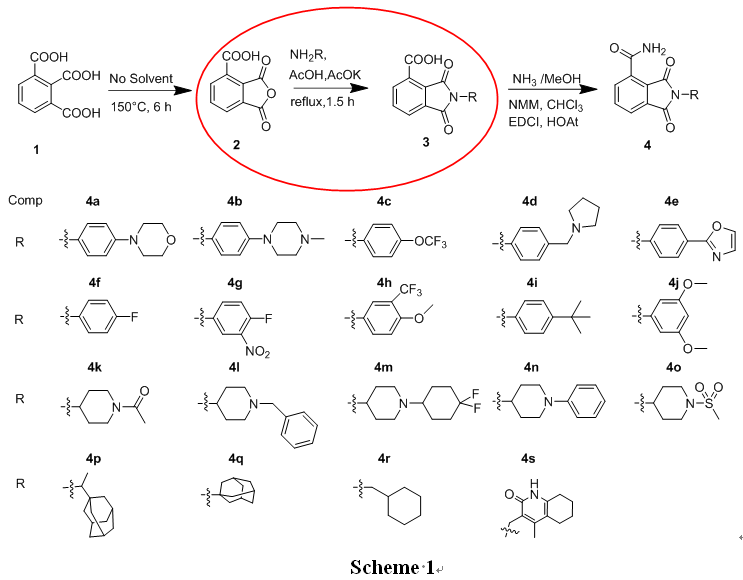
N- substituted phthalimide -4- formamide mother core is a brand-new skeleton as PARP-1 inhibitor. Professional chemical manufacturers take 1,2,3- phthalic acid (1) as raw material, and carry out intramolecular dehydration under heating conditions to obtain 3- carboxylic phthalic anhydride (2). 2 were aminated with different substituted primary amines to obtain N- substituted phthalimide -4- carboxylic acid derivatives (3a ~ 3s). N- substituted phthalimide -4- formamide (4a~4s) was obtained by amide condensation reaction between the latter and ammonia.
The anti-cancer activity of compound 4 on pancreatic cancer cell line Capan-1 and breast cancer cell line MDA-MB-231 was studied by MTT method, and the results showed that compound 4r: capan-1 had a 50% inhibitory concentration of 0.19μM, which was better than NMS-P118 and equivalent to Olaparib. This series of compounds also showed inhibitory effect on Brca2 mutant cell line, which indicated that they have further development value as PARP-1 inhibitors.